MiRus® Receives FDA 510(k) Clearance for
CYGNUS MoRe® Anterior Cervical Plate
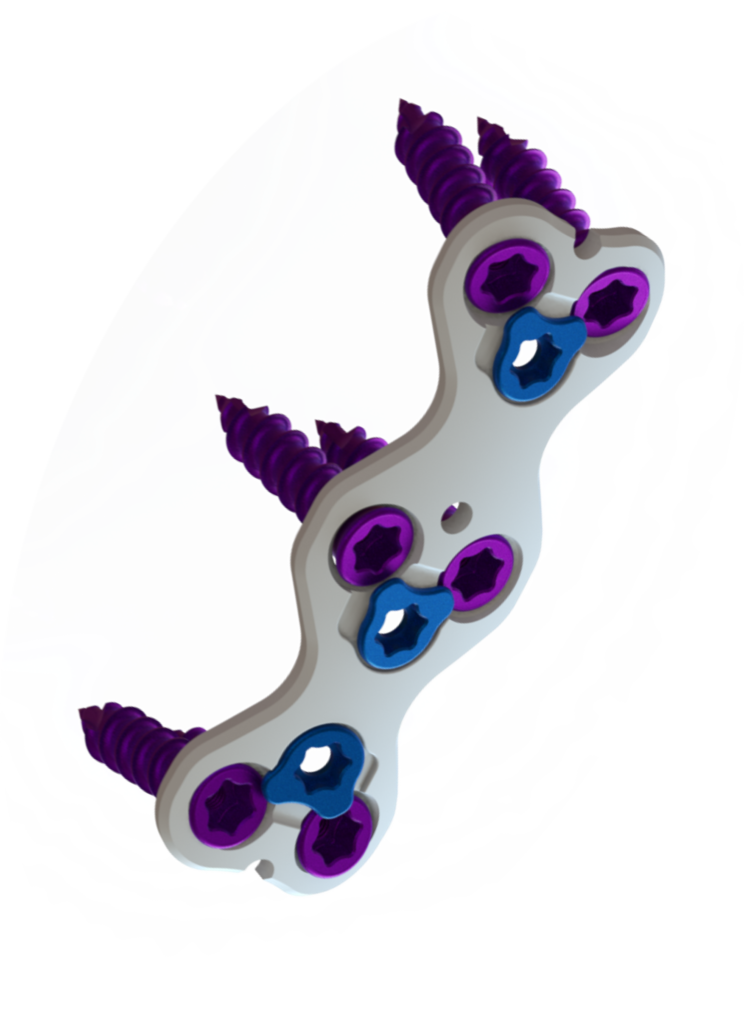
ATLANTA — August 15, 2022— MiRus announced today that it has received FDA 510(k) clearance of the CYGNUS MoRe® Anterior Cervical Plate System, the narrowest and thinnest cervical plate on the market. The molybdenum-rhenium alloy used in the plate allows for a dramatically smaller footprint of 1.0mm thickness without sacrificing performance. The CYGNUS MoRe® Anterior Cervical Plate with its narrower and thinner profile will lead to less retraction and anterior vertebral body preparation resulting in shorter operative times and minimizing risk of complications such as dysphagia and dysphonia in patients undergoing anterior cervical fusion.
“The CYGNUS MoRe® Anterior Cervical Plate System is another major accomplishment as we strive to develop products that are best in class with resolve to improve patient outcomes and provide surgeons with premium products. The CYGNUS MoRe® ACP is a world class product in the ACDF marketplace that furthers our commitment to minimally invasive, superior strength biofriendly implants” remarked Mahesh Krishnan, Chief Commercial Officer.
About MiRus®, LLC.
MiRus is a life sciences company headquartered in Marietta, Georgia that has developed and is commercializing proprietary novel biomaterials, implants and procedural solutions for the treatment of spine, orthopaedic and structural heart disease. Find out more information about MiRus at www.mirusmed.com.
Statements made in this press release that look forward in time or that express beliefs, expectations or hopes regarding future occurrences or anticipated outcomes are forward-looking statements. A number of risks and uncertainties such as risks associated with product development and commercialization efforts, expected timing or results of any clinical trials, ultimate clinical outcome and perceived or actual advantages of the Company’s products, market and physician acceptance of the products, intellectual property protection, and competitive offerings could cause actual events to adversely differ from the expectations indicated in these forward looking statements.
CYGNUS™ , MoRe® and MiRus® are trademarks of MiRus, LLC.